Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is the standard treatment for patients younger than 40 years with Severe and Very Severe Aplastic Anemia (AA) who have a Matched Related Donor (MRD). For patients lacking a MRD, treatment options include immunosuppressive therapy (IST), matched unrelated donor (MUD) or alternate donor (haploidentical/cord blood) transplant. Pakistan has a population of around 23 million but there is no donor registry for MUD transplants and horse antithyomcyte globulin (hATG) is not available. Over past decade, results of haploidentical transplants have improved remarkably with use of post-transplant cyclophosphamide. However, most of these protocols incorporate total body irradiation (TBI) to improve engraftment and reduce graft failure. TBI is not available in most of the transplant centres across Pakistan. We have developed a novel TBI free conditioning regimen (NCT03955601) for haploidentical HSCT in acquired AA patients lacking a MRD.
Materials and Methods
We conducted a prospective, single centre, interventional trial (NCT03955601) using novel TBI free conditioning at AF bone marrow transplant centre (AFBMTC)/ National institute of blood and marrow transplant (NIBMT) for patients with acquired severe and very severe AA. Between July 2018 and March 2020, a total of 10 patients received related haploidentical transplant.Study inclusion criteria was diagnosis of severe and very severe AA, patients of both genders, age 2-60 years, Karnofsky performance status>70%. Exclusion criteria was presence of donor specific antibodies (DSA), inherited bone marrow failure syndrome, prior HSCT and severe sepsis. Conditioning regimen used was Fludarabine (Flu) 30 mg/m2 IV daily from day -7 to -3, Cyclophosphamide (Cy) 14.5 mg/kg IV daily on day -6 and -5 , rabbit Antithymocyte globulin (rATG) 5 mg /kg/day from day -6 to day-3; Busulphan (Bu) IV 3.2 mg per kg/day in 04 divided doses on day -3 and day-2, Granulocyte Colony Stimulating factor (GCSF) primed Bone marrow harvest (BMH) and/or PBSC graft on day 0 and day +1 respectively. Graft versus host disease (GVHD) prophylaxis used was post-transplant cyclophosphamide (PTCy) administered at a dose of 50mg/kg/day given daily on days +3 and +5 post-transplant, cyclosporine (CsA) from day +5 and mycophenolate mofetil (MMF) from day+5 to +35. Primary outcome measure was overall survival (OS) while secondary outcome measures included graft failure, treatment related mortality (TRM), disease free survival (DFS), GVHD free relapse free survival (GRFS), acute and chronic GVHD.
Results:
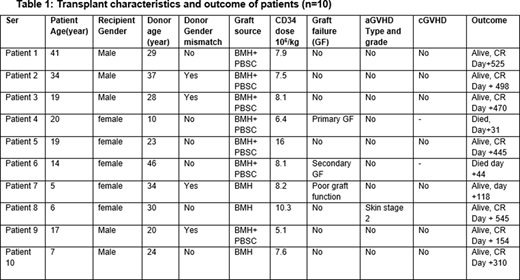
Ten patients were transplanted, 5 (50%) female and 5 (50%) male (table 1). Median age was 15.5 years (range 5-41 years). Median duration from diagnosis to transplant was 14 months (range 4-51 months). One patient received ATG prior to transplant. Median number of red cell concentrate (RCC) transfusions before transplant were 27 (8-90) and platelet transfusions 100(6-150). Median donor age was 23 years (10-41 years). Donor-recipient major ABO mismatch was present in 2(20%) while four (40%) had minor ABO mismatch. Primed bone marrow harvest (BMH) was used in 3(30%) while primed BM+PBSC was given in 7(70%) patients. Median CD34 dose given was 8 x 106/kg (range 5.1-16). Seven patients (70%) achieved sustained neutrophil engraftment. One patient had primary graft failure, one patient secondary graft failure at day 35 due to Cytomegalovirus infection and one patient (currently day +118) is having poor graft function. Acute skin GVHD stage-2 developed in 1 patient which settled with topical steroids. None of the patient developed chronic GVHD. Cytomegalovirus reactivation was documented in 8 (80%) patients. All donors and recipients were CMV seropositive pre-transplant. One patient (female, 20 years) had primary graft failure and died on day +31 with sepsis and multiorgan dysfunction syndrome (MODS). One patient (female, 14 years) had secondary graft failure due to CMV infection and died on day +44 with intracranial hemorrhage. Conditioning regimen was well tolerated without any treatment related morbidly and mortality. Median follow-up of study was 13 months (range 4-22 months). Overall survival of study cohort is 80%, DFS 70% and GRFS 70%.
Conclusion:
Our study shows that for countries lacking TBI, use of FluCAB-Prime protocol is feasible and is associated with low rates of acute and chronic GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal